Past Issues
Phase Behavior of Low-Temperature Metallomesogen and Commercial Liquid Crystal Mixtures
Hassan-Ali Hakemi*
Plastic Liquid Crystal Technology, Via Lambro 80, 20846 Macherio (MB), Italy
*Corresponding Author: Dr. Hassan-Ali Hakemi, Plastic Liquid Crystal Technology, Via Lambro 80, 20846 Macherio (MB), Italy, Phone: +39 349 5679838; Email: [email protected]
Received Date: June 14, 2024
Publication Date: July 30, 2024
Citation: Hakemi HA. (2024). Phase Behavior of Low-Temperature Metallomesogen and Commercial Liquid Crystal Mixtures. Material Science. 6(2):30.
Copyright: Hakemi HA. © (2024).
ABSTRACT
We studied the effect of a low-temperature nematic metallomesogen (MOM) model structure based on synthesized rod-like Palladium (Pd) Alkyl/Alkoxy-Azobenzeneon on transition temperatures of its eutectic ligand/MOM mixture with a low-temperature E43 and high-temperature TN10427 commercial nematic liquid crystals. The results indicate complete and wide-range nematic miscibilies of these model mixtures as potential materials for application in electro-optical devices.
Keywords: Metallomesogen, Ligand, Low-Temperature, Phase Diagram, Nematic, Miscibility, Eutectic Point
INTRODUCTION
The metal-organic liquid crystals known as "metallomesogens" (MOMs) consisting of metal- complex centers in organic mesogenic structures have been studied for decades as potential materials for technological applications [1-7]. The presence of metal complexation in the chemical structures of liquid crystals adds many physical and optical features that are not present in the organic mesogenic systems. These features have been the main driving force for potential application of MOMs in a wide range electro-optical device applications by combining the mesogenic supramolecular order and additional properties of metal-complexation.
In spite of many developments regarding the chemistry and phase behavior of varieties of synthsized MOMs, there have been few recent studies on their applications in electro-optical devices, smart sensors, encryption systems and fuel cells [1,8], photo-luminescence and water-free proton conduction [10-12], electro-luminescence [9,13], magnetic [14,15] and electric [16-18] properties. In addition, there have been further scientific [19-23] and patent [24-28] literature on potential applications of calamitic and discotic MOMs as dichroic dyes, non-linear optics, thermal recording, thermo-chromism, passive optical filters, photo-sensing, laser addressing, optical and thermal recording, PDLC and polarizing flms, radiation absorbing films, ferroelectricity, ferromagneticity, electroconductivity, reaction catalysts, liquid crystal intermediates, ink jet and security printing, medicinal and agricultural components.
However, in spite of extensive literature on MOMs, such materials have not yet been developed for commercial application. In fact, the major drawbacks of studied MOMs have been due to their high transition temperatures, small mesophase stabilities and risk of chemical decomposition. In order to develop MOMs for commercial application, one requires providing qualified materials with new properites not found in organic liquid crystals. Therefore, in addition to stable chemical structure, the appropriate solution for commercial application of MOMs is their accessible mesogenic transition temperature, which could be obtained through their mixings and miscibitities similar to commercial liquid crystal materials. Except to our previous and recent studies on physical and chemical mixtures of MOMs [29-33], we are not aware of any other literature works on the MOMs’ mixing approach.
In this work, we utilized a low-temperature mono-ligand MOM model structure based on Palladium alkyl/alkoxy-azobenzene complex and studied the transition temperatures and phase diagrams of a binary MOM/ligand mixtures. Subsequently, with mixed the eutectic composition of this low- temperature mixture with two commercial E43 and TN10247 liquid crystals as potential materials for application. The results of these studies are provided in the following sections.
MATERIALS AND METHODS
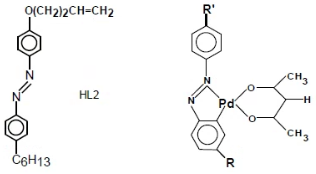
The synthesized HL2 ligand and HL2/Pd-acac MOM were based on a common class of mono-ligand palladium alkyl/alkoxy-azobenzenes metal-complexes, which are presented in the following general chemical formula.
The original synthetic procerdures of ligand and MOM chemical structures have been reported elsewhere [29,30]. Accordingly, the chemical structures of MOM were obtained by incorporating its parent ligand in Pd-alkyl/alkoxy-azobenzene complex having the following chemical compositions:
- : R= C6H13; R'= O(CH2)2CH=CH2
- : R= C6H13; R'= O(CH2)2CH=CH2
Further details on the synthetic procedures this class of ligand and mono-ligand MOM compounds have been mentioned elsewhere [29,34,35]. The studied transition temperatures of HL2 ligand, HL2Pd-acac MOM, E43, TN10427 materials consisted of crystal-mesogenic (Tcm) and mesogenic- isotropic (Tmi) on heating mode, as well as the isotropic-mesogenic (Tim) and mesogenic-crystal (Tmc) transition temperatures on cooling mode, were determined by a Perkin Elmer DSC7 Differential Scanning Calorimeter (DSC) method. In addition, the phase transitions and mesophase types of the components and mixtures were carried out by Nikon Eclipse-50i polarizing optical microscope (POM) equipped with temperature controlled Mettler FP5 microscopic hot-stage.
The phase diagrams of the mixtures were carried out by direct weighing of the components in DSC pan and through the repeated DSC scanning at heating rate of 10 °C/min and cooling rate of 5 °C/min until the mixings were completed with no change in their thermograms. The same DSC mixtures were then utilized to determine their liquid crystalline phases by POM method.
RESULTS
In Table-1, we tabulate the transition temperatures on cooling mode of synthesized low-temperature L2 ligand, L2Pd-acac MOM, where both exhibit mesogenic stabilities below 70°C. With respect to mesomorphism, the HL2 ligand exhibits entantiotrpic nematic phase and 33°C nematic stability range, whereas HL2Pd-acac exhibits monotropic nematic phase and nematic stability range of 55°C. The results of the phase diagrams of binary HL2/HL2Pd-acac mixtures and its eutectic point composition in ternary mixtures with commercial liquid crystals E43 and TN10427 are described as follows.
Table 1. The transition temperatures of ligand, MOM and commercial liquid crystals
|
Compound |
Transition Temperature (°C) |
Mesophase |
|||
|
Heating |
Cooling |
||||
|
Tcm |
Tmi |
Tim |
Tmc |
||
|
L2 |
41.7 |
51.1 |
48.1 |
14.8 |
Enantiotropic Nematic |
|
L2Pd-acac |
66.2 |
- |
43.1 |
-12 |
Monotropic Nematic |
|
E43 |
- |
- |
78 |
-30 |
Enantiotropic Nematic |
|
TN10427 |
- |
- |
114 |
-40 |
Enantiotropic Nematic |
L2 Ligand and L2Pd-acac MOM Mixtures
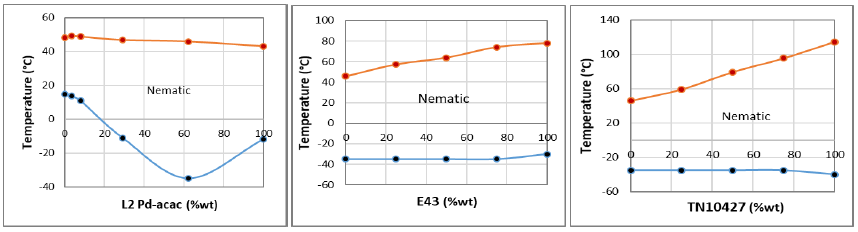
As a model system, we studied the mixtures of low-temperature HL2 ligand and HL2Pd-acac MOM having the same R and R’ terminal groups. Accordingly, with reference to Table-1and Figure-1 (left), we present the transition temperatures and phase diagram of HL2/HL2Pd-acac mixtures on cooling mode. It is noticed that due to linear trend of isotropic-nematic (TIN) transitions, the low-temperature mixtures exhibit a complete nematic miscibility within the whole composition of the phase diagram.
Also, both HL2 ligand and HL2Pd-acac MOM exhibit similar TIN range, where the presence of Pd metal-complex in L2Pd-acac exhibits a wider nematic range (55.1°C) than L2 ligand (33.3°C). Also the nematic-crystal (TNC) transitions of this mixture exhibits a distinct eutectic point at L2Pd-acac = 62.3%wt composition. At this eutectic point, the nematic range of the mixture is widely expanded to 81°C (i.e., from TIN=+45.9°C to TNC=-35°). Such a wide low-temperature nematic range at the eutectic point of this model mixtures suggests that such systems could be considered as potential material for application in the electro-optical devices.
Eutectic L2/L2Pd-acac and Commercial Liquid Crystal Mixtures
In order to evaluate the eventual utilization of MOMs for eventual application, we also studied the transition temperatures and phase diagram of eutectic HL2/HL2Pd-acac (37.7/62.3%wt) with commercial low-temperature E43 (Merck) and high-temperature TN10427 (Hofmann La Roche) nematic mixtures.
According to Figure-2 (left), we provide the transition temperatures and phase diagram of HL2/HL2Pd-acac / E43 mixtures on cooling mode. The linear trend of TNI transion temperatures clearly demonstrate the complete miscibility of nematic phase within the whole composition range of HL2/HL2Pd-acac / E43 phase diagram. Also, as both components are eutectic, their TNC transition temperatures exhibit a linear trend within the total compositions of phase diagram. The stability of nematic phase in this low-temperature system is within 81-108 °C, which is sufficiently wide for application at various compositions of their phase diagram.
In Figure-2 (right), we provide the transition temperatures and phase diagram of eutectic HL2/HL2Pd- acac (37.7/62.3%wt) with commercial high-temperature nematic TN10427 mixtures on cooling mode. The phase diagram of this mixture also demonstrates the total miscibility of nematic phase within the total concentration range of the phase diagram due to linear trend of TNI transion temperatures. Also, the TNC transition temperatures of HL2/HL2Pd-acac / TN10427 mixtures also exhibit a linear trend, where the nematic stability in this mixture is quite wide and lies within 81-154 °C range at total compositions of the phase diagram.
Figure 1. Phase diagrams of (left) HL2/HL2Pd-acac; (center) HL2/HL2Pd-acac/E43 and (right) HL2/HL2Pd-acac/TN10427.
DISCUSSION
In the present study, we provided a model low-temperature mono-ligand MOM structure based on Pd- alkyl/alkoxy-azobenzene complex. Accordingly determined its phase diagram and transition temperatures in binary mixtures with parent ligand, utilized their eutectic composition and studied the ternary mixtures of its phase diagram with two commercial nematic liquid crystals. Subsequently, we suggested that it is possible to introduce MOMs as alternative materials for potential application in elecro-optical devices, providing that other properties of mesogenic metal-complexes not present in organic liquid crystals will be exploited. A brief discussion of the results are as follows:
- The rod-like HL2 ligand and HL2Pd-acac MOM with the same terminal groups exhibited low transition temperatures and accessible mesogenic stabilities of 33°C and 55°C, respectively. The presence of Pd metal complex in the HL2Pd-acac MOM expanded its nematic phase by 22°C larger than that of HL2 ligand
- The phase diagram of low-temperature HL2 and HL2Pd-acac binary mixtures showed complete nematic miscibility within the whole composition range of phase diagram and exhibited distinctive eutectic point at HL2Pd-acac=62.3% composition and a wide nematic stability range of 81°C.
- The ternary mixtures of eutectic HL2/HL2Pd-acac with commercial low-temperature E43 and high-temperature TN10427 also exhibited total nematic misciblity and wide nematic stability up to 81°C (E43) and 154°C (TN10427) range within the total compositions of their phase diagram. The phase behavior of these two model systems further indicates that introduction of MOMs in commercial liquid crystal products is a viable approach for potential application.
CONCLUSION
The results of this preliminary study demonstrated that eutectic ligand/MOM mixture could be considered as potential guest in commercial liquid crystal host materials. However, the present model MOM system is not the final solution and their eventual commercial application requires future systematic developments to obtain qualified chemical structures and performances, such as stable chemical structures, wide and accessible mesogenic stability, complete mesogenic miscibility with commercial liquid crystals and exploitation of other MOMs’ physical and optical properties not present in the organic liquid crystals.
ACKNOWLEDGMENTS
The author would like to thank the Snia Riceche - Snia BPD (Fiat Group), who sponsored the Electro-Optical Film Group project on metallomesogen research and development project under collaboration with Professor M. Ghedini’s group at the University of Calabria, Rende (CA) Italy, during 1993-1996 period.
REFERENCES
- Serrano JL. (1996). Metallomesogens: Synthesis, Properties and Applications, Wiley VCH, New York, USA.
- Donnio B, Bruce DW. (1999). Liquid Crystals II Metallomesogens. Mingos DMP, editor. Vol. 95, Springer, Germany.
- Donnio B, Guillon D, Deschenaux R, Bruce DW. (2003). Comprehensive Coordination Chemistry II. V6, McCleverty JA, Meyer TJ, editors, Elsevier Oxford, UK.
- Date RW, Iglesias EF, Rowe KE, Elliott JM, Bruce DW. (2000). Dalton Transactions. pp. 1914-1931.
- Bruce DW, Deschenaux R, Donnio B, Guillon D. (2006). Comprehensive Organometallic Chemistry III. In: Crabtree RH, Mingos DMP, editors, Elsevier Oxford UK. pp. 195.
- Bruce DW. Liquid Crystals. Available at: https://doi.org/10.1080/02678292.2023.2245362
- Porta B, Khamsi J, Noveron JC. (2008). Curr Org Chem. V12. pp. 1298.
- Wang Y, Shi J, Chen J, Zhu W, Baranoff E. (2015). Recent progress in luminescent liquid crystal materials: design, properties and application for linearly polarised emission. Journal of Materials Chemistry C. 3(31):7993-8005.
- Wang Y, Fan J, Shi J, Qi H, Baranoff E, Xie G, et al. (2016). Influence of integrated alkyl-chain length on the mesogenic and photophysical properties of platinum-based metallomesogens and their application for polarized white OLEDs. Dyes Pigments. 133:238-247.
- Krikorian M, Liu S, Swager TM. (2014). Columnar Liquid Crystallinity and Mechanochromism in Cationic Platinum(II) Complexes. J Am Chem Soc. 136(8):2952-2955.
- Geng H, Luo K, Cheng H, Zhang S, Ni H, Wang H, et al. (2017). Novel columnar metallomesogens based on cationic platinum(ii) complexes without long peripheral chains. RSC Adv. 7(9):11389.
- Cristián Cuerva de Alaíz, PhD thesis, 2018, Department of Inorganic Chemistry, University of Madrid.
- Liu SH, Lin MS, Chen LY, Hong YH, Tsai CH, Wu CC, et al. (2011). Polarized phosphorescentorganic light-emitting devices adopting mesogenic host–guest systems. Org Electron. 12(1):15-12.
- Seredyuk M, Muñoz MC, Ksenofontov V, Gütlich P, Galyametdinov Y, Real JA. (2014). Spin Crossover Star-Shaped Metallomesogens of Iron(II). Inorg Chem. 53(16):8442-8454.
- Fitzpatrick AJ, Martinho PN, Gildea BJ, Holbrey JD, Morgan GG. (2016). Robust Room Temperature Hysteresis in an FeIII Spin Crossover Metallomesogen. Eur J Inorg Chem. 2016(13-14):2025.
- Ionescu A, Godbert N, Crispini A, Termine R, Golemme A, Ghedini M. (2012). Photoconductive Nile red cyclopalladated metallomesogens. J Mater Chem. 22(44):23617-23626.
- Su PYS, Tseng JCW, Lee KM, Wang JC, Lin IJB. (2014). Advanced molecular materials based on novel Pd(II) and Pt(II) metallomesogens for technological applications. Inorg Chem. 53:5902.
- Su PYS, Hsu SJ, Tseng JCW, Hsu HF, Wang WJ, Lin IJB. (2016). Polynuclear Silver(I) Triazole Complexes: Ion Conduction and Nanowire Formation in the Mesophase. Chem Eur J. 22(1):323-330.
- Ginord-Godquin M, Maitlis PM. (1991). Metallomesogens: Metal Complexes in Organized Fluid Phases. Angew Chem Int Engl. 30(4):375-402.
- Bruce DW. (1992). In: Bruce DW & Hare DO, (Eds.). Inorganic Materials. J Wiley & Sons Ltd, New Jersey, United States.
- Blanca Ros M. (1996). Other Physical Properties and Possible Applications of Metallomesogens. In: Serrano Jl, (Eds.). Metallomesogens - Synthesis, Properties & Applications. pp. 498.
- Gimenez R, Lydon DP, Serrano JL. (2002). Metallomesogens, a Promise or a Fact? Current Opinion in Solid State and Materials Science. 6(6):527-535.
- Binnemans K. (2010). Inorganic Materials Series. In: Bruce DW, O’Hare D, Waton RI, editors. J Wiley. pp. 61-133.
- Hakemi H. WO 95/01410.
- Hakemi H, Caporusso M, Santangelo M. EP 0747 461 A1.
- Roviello R, Centore B, Panunzi, Hakemi H. IT1394422.
- Hakemi H, Lofer A, Peso E. US62/065,805; PCT47459 WO2016/06327.
- Hakemi H, Lofer A, Peso E, Gal-Fuss D. US62/076,002.
- Hakemi H, Roviello A, Sirigu A, Panunzi B, Ghedini M. (2002). Proceedings of 19th ILCC- Edinburgh, UK.
- Hakemi H. (2022). Metallomesogen Mixtures as Potential Materials for Application in Liquid Crystal Devices. J mate poly sci. 2(4):1-5.
- Hakemi H, Roviello V, Caruso U. (2023). Inorganics. 11(1):32.
- Hakemi H. (2023). Mat Sci & Eng Intern J. 7(2):108.
- Hakemi H. (2024). New Metallomesogen Chemical Mixtures by In-Situ One-Pot Chemical Solution Synthesis Method. J Mater sci Appl 8:1-7.
- Ghedini M, Pucci D, Neve F. (1996). Mesogenic cyclopentadienyl cyclopalladated azobenzene complexes. Chem Commun. 1996(2):137-138.
- Ghedini M, Morrone S, Neve F, Pucci D. (1996). Gazz Chim. 126:511.
 Abstract
Abstract  PDF
PDF