Past Issues
Antibacterial Activity of Nanosheets and Nanoflowers in Molybdenum Disulfide: A Comprehensive Review
Mohammad Hossein Karami*, Behzad Aghabarari
Nanotechnology and Advanced Materials Department, Materials and Energy Research Center, Karaj 31787-316, Iran
*Corresponding Author: MohammadHossein Karami, Nanotechnology and Advanced Materials Department, Materials and Energy Research Center, Karaj 31787-316, Iran; Email: [email protected]
Received Date: May 28, 2024
Publication Date: June 18, 2024
Citation: Karami MH, et al. (2024). Antibacterial Activity of Nanosheets and Nanoflowers in Molybdenum Disulfide: A Comprehensive Review. Material Science. 6(1):28.
Copyright: Karami MH, et al. © (2024).
ABSTRACT
Untreated or persistent wounds have a detrimental impact on patient well-being and place a substantial strain on global public health. Reactive oxygen species (ROS) are pivotal in impeding the healing of wounds. Ailments pose a significant hazard to human health. Inappropriate use of antibiotics can weaken the immune system and result in numerous negative effects on the human body. Antibacterial agents based on nanomaterials represent a promising method to prevent infections and inhibit bacterial growth. Recent studies have emphasized molybdenum disulfide (MoS2) as an exceptional member of transition metal dichalcogenides (TMDs) due to its expansive surface area, strong near-infrared (NIR) absorption, high biocompatibility, and low toxicity towards cells. MoS2-based nanomaterials have displayed efficacy in inhibiting bacterial growth across various systems. This review aims to present an outline of recent research on the antibacterial attributes of MoS2-based nanomaterials, encompassing MoS2 nanosheets, nanoflowers, cytotoxicity, innovative nanosystems, and quantum dots (QDs).
Keywords: MoS2, Nanosheets, Nanoflowers, Cytotoxicity, Novel Nanosystems, Quantum Dots
INTRODUCTION
More and more people are falling ill due to germs such as bacteria and viruses, leading to millions of deaths worldwide. Bacterial infections result from germs entering the body through cuts or the airway, triggering specific reactions within our bodies [1]. Bacterial infections pose a significant threat to society as they can result in numerous deaths and immense suffering. The most effective way to treat these infections is with appropriate antibiotics [2].
Getting treatment for bacterial infections is very important as untreated infections can lead to serious health problems. Many bacterial illnesses can be effectively treated with antimicrobial drugs. These drugs function by disrupting crucial processes within the bacteria's cells, such as their cell walls, DNA, and proteins. However, the effectiveness of traditional antibiotics is diminishing due to the increasing resistance of bacteria, leading to high treatment costs [3]. The misuse of antibiotic drugs is a growing issue, resulting in various side effects [4].
Also, some studies have shown that excessive antibiotic use has led to the emergence of bacteria that are resistant to multiple drugs. Therefore, it is crucial to utilize advanced antibacterial materials to combat these issues. Nanomaterials are currently being extensively utilized in the fields of business and medicine. At the nanoscale level, materials exhibit unique properties compared to their larger forms, primarily due to their small size, shape, and high surface area-to-volume ratio [5]. As a result, nanomaterials may serve as a promising alternative to conventional antibiotics in addressing bacterial resistance [6].
Nanomaterials are more effective at killing bacteria than regular materials due to their unique properties. Carbon-based materials, small pieces of metal, metal compounds, and modified plastics are being used as antibacterial materials in minute sizes [7]. Non-biological tiny particles interact with germs in a highly effective manner. Nanoparticles contain specific components that can exterminate bacteria by producing chemicals that react with oxygen and discharge metal particles. Composite materials consisting of metal oxides are beneficial for the environment as they are affordable, stable at high temperatures, and non-toxic to humans [8].
Kannan and his colleagues showed a mixture of special earth-based metal oxide nanohybrids and tested their effectiveness in killing bacteria. The nanocomposites demonstrated an exceptional ability to eliminate bacteria, making them suitable for use in chemical and environmental applications [9].
The use of 2D nanomaterials like MXene, graphene, h-BN, and TMDs for antibacterial purposes is increasing. These materials have a large specific surface area and surface modifications that enhance their adherence to bacterial membranes. By functionalizing these nanomaterials, they can effectively interact with bacterial membranes, thereby enhancing their antibacterial properties [10].
Unlike conventional antibiotics, antibacterial agents made from 2D nanomaterials can be utilized in smaller quantities, reducing the risk of side effects and resistance issues. TMDs such as MoS2 show promise in combating cancer and bacteria in the medical field due to their mobility, stability, affordability, compatibility with the body, versatility, and ease of production [11]. Researchers are exploring various applications of MoS2, including enhancing performance, medical uses, and electronics. While MoS2 nanomaterials possess significant attributes, there are limitations when using them in medicine without proper modification. Enhancing MoS2 by incorporating additional functionalities can expand its potential applications. Additionally, combining MoS2 with other antibacterial materials can greatly enhance its effectiveness [12].
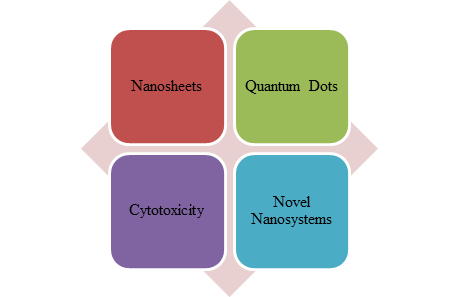
The research on assessing the antibacterial properties of MoS2-based materials is rapidly expanding, making it a crucial area of study at present. This review aims to provide an overview of the latest research on the antibacterial properties of MoS2-based nanomaterials, including MoS2 nanosheets, nanoflowers, cytotoxicity, novel nanosystems, and quantum dots (QDs)(Fig.1).
Figure 1: Important findings regarding the antibacterial properties of MoS
Toxicity of MoS2 based nanomaterials
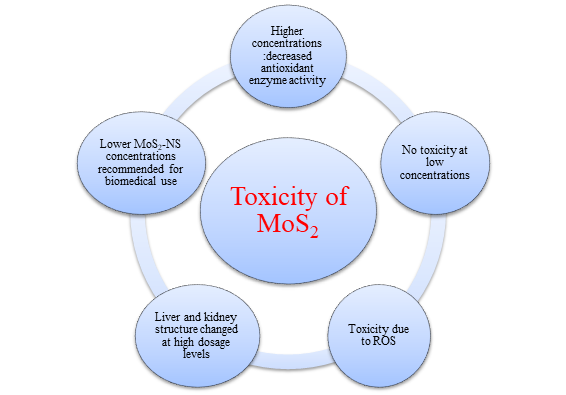
The increased use of MoS2-based nanomaterials in various applications poses a threat to the environment and human health. A study found that larger quantities of MoS2 are more harmful than thin sheets of the material. Different organisms were tested, and it was found that Daphnia magna, a type of aquatic creature, was the best test organism for evaluating the effects of MoS2 [13]. Another study showed that MoS2 film and microparticles have toxic effects on cells, but MoS2 thin films are safe for cell use at low concentrations. In testing on guinea pigs, the impact on the animals' skin was minimal. MoS2 nanosheets were found to be more harmful to soil bacteria compared to bulk MoS2 [14]. They were also less effective at killing bacteria compared to other antibacterial medications. The extraction process of MoS2 was found to affect its toxicity, with certain chemicals causing less harm to cells. Ingesting small particles of MoS2 led to oxidative stress and altered behavior in Asian weaver ants. However, MoS2 did not significantly harm human macrophages or liver cancer cells. It was also found that MoS2, WS2, and TiS2 accumulate in the body's immune organs after administration [15]. Amazingly, the MoS2-PEG was completely eliminated from the body after 30 days. The mouse's organs contained more titanium or tungsten when they were exposed to TiS2-PEG or WS2-PEG. However, when TiS2 is exposed to oxygen, it transforms into TiO2. TiO2 is insoluble in water and is challenging to eliminate from the body. WS2 resists breaking down when exposed to chemicals, resulting in prolonged retention in the body. Figure 2 presents the main findings from experiments conducted in animals and in a laboratory setting [16].
Figure 2: Analyzing the cytotoxic effects of MoS2 nanoparticles
Antibacterial studies in MoS2 based nanomaterials
Nanomaterials are being increasingly used to kill bacteria due to their large surface area, ability to penetrate layers, adjustable surface, compatibility with living tissues, and antibacterial properties. These properties enable them to effectively kill bacteria with smaller amounts of medicine, reducing side effects. The antibacterial action of 2D nanomaterials is caused by physical damage, oxidative stress, and light-induced processes [17]. The process of killing bacteria involves applying nanomaterials to the cell wall, disrupting the membrane, and inhibiting the bacteria's functions and structure. The sharp edges of nanosheets can harm the cell membrane, leading to leakage and death of microorganisms. Oxidative stress is another mechanism that inhibits bacterial metabolism and kills bacteria [18]. ROS can severely damage bacteria, breaking down the cell wall and allowing harmful substances to penetrate. Excessive ROS can also damage cell pathways, impacting crucial components of the cell or microbial structure. Light-activated methods, such as photodynamic, photocatalytic, and photothermal, can be used to eliminate bacteria with fewer side effects and target specific areas [19]. Graphene and MoS2 are extensively used in medical applications, with graphene-based materials having various uses in delivering medicine, imaging, disease treatment, immune system sensors, and the food industry. MoS2, with its structure and chemical composition, also possesses antibacterial properties through physical contact, oxidative stress, and exposure to light. Overall, nanomaterials and light-activated substances offer promising strategies for killing bacteria effectively and reducing side effects [20].
MoS2 nanosheets
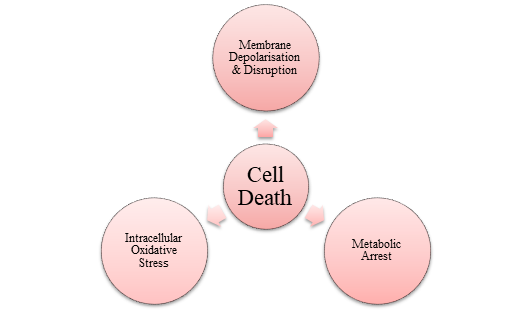
The use of MoS2 in killing bacteria is gaining importance, especially when using smaller nanosheets that can harness light for the process. Thinner and smaller MoS2 nanomaterials can efficiently break down pollutants using light and inhibit bacterial growth. Even under normal light conditions, the production of ROS can effectively kill bacteria [21]. 1T MoS2 has better conductivity for carrying electrons, making it more suitable for reactions when exposed to light, unlike 2H MoS2. Chemically exfoliated MoS2 nanosheets show antibacterial properties, including producing ROS and inducing oxidation. Ce-MoS2 nanosheets have stronger antibacterial effects due to their larger surface area and open edges, allowing for a greater interaction with bacteria and more efficient electron exchange [22]. Higher concentrations of MoS2 nanosheets lead to cell membrane destruction and decreased bacteria viability, while lower concentrations increase intracellular metabolites [23]. Using chitosan, MoS2 nanosheets can effectively kill bacteria by damaging their outer layer, halting their metabolism, and inducing oxidative stress within the cells. However, the mechanism behind MoS2 nanosheets' antibacterial abilities without external forces or antibiotics is not well understood. Figure 3 depicts the main points from both in vivo and in vitro analyses [24].
|
|
Figure 3: The purpose of the antibacterial activity of CS-MoS2 nanosheets
CS-MoS2 nanosheets have shown good compatibility with mammalian cells and efficacy against biofilm. The antibacterial and anti-biofilm properties of chitosan-exfoliated MoS2 nanosheets need to be evaluated without the use of additional substances [25]. Altering the synthesis methods can impact the morphology and structure of MoS2, giving different properties. In their study, Zhang's team experimented with MoS2 using ultrasound treatment, intercalation, and liquid phase exfoliation [26]. They found that MoS2 produced via intercalation (IN-MoS2) had potent sterilization capabilities, smaller particle size, and higher metal content compared to the other methods. IN-MoS2 generated more oxygen species due to enhanced electron transfer and improved separation of light-induced charge carriers. Gel electrophoresis showed that IN-MoS2 caused significant DNA damage [20]. In another study, Kumar and his team developed surgical masks with a layer of MoS2 nanosheet-modified polycotton fabric, which had photothermal and excellent antibacterial properties. The fabric showed complete self-disinfection in sunlight and maintained its antibacterial function after multiple washing cycles. The antimicrobial activity was attributed to oxidative stress and surface-contact-mediated membrane disruption [27].
MoS2 nanoflowers
A recent investigation has compared the antibacterial potency of MoS2 nanosheets with that of MoS2 nanoflowers. The researchers found that the larger surface area of the nanoflowers allowed for greater contact with bacteria, resulting in higher bacteria-killing abilities compared to the nanosheets. The absence of superoxide anion production indicated that the antibacterial mechanism relied on ROS. Additionally, the nanoflowers demonstrated a higher capability to oxidize GSH [28]. A study was conducted to investigate the antibacterial properties of 2H-MoS2 and 1T-MoS2 nanoflowers when exposed to light. 1T-MoS2 nanoflowers showed strong antibacterial efficacy by generating superoxide anion radicals when exposed to light. In contrast, recombination in 2H-MoS2 nanoflowers decreased ROS production and antibacterial activity [29].
MoS2, which mimics peroxidase, has the potential to be used in treating cancer and has shown efficient conversion of light into heat. Researchers developed a germ-killing system using MoS2 nanoflowers treated with polyethylene glycol, which enhanced its biocompatibility and germ-killing capabilities. PEG-MoS2 nanofibers demonstrated bacteria-killing abilities without relying on ROS [30]. TiO2 nanotubes coated with MoS2 nanoflowers exhibited high effectiveness in treating bacteria by enhancing their water-cleaning abilities. Further investigation is needed to explore and enhance the antibacterial properties of MoS2 nanomaterials using appropriate chemicals or materials [31].
MoS2 quantum dots
Changing the positioning of the band hole in MoS2 nanomaterials can enhance their ability to utilize light for chemical reactions. This indicates that reducing the size and thickness of MoS2-based nanomaterials can increase their capacity to initiate chemical reactions through light absorption. MoS2 quantum dots (QDs) are minute fluorescent materials with unique light properties that are being researched for their benefits in biological applications [32-35]. MoS2 QDs are ideal for implementing in photocatalytic processes due to their ability to carry various charges, possess multiple facets, and offer a large surface area [20].
Although 2D MoS2 nanosheets are effective in combatting bacterial infections, there is limited exploration on the antibacterial attributes of MoS2 QDs. In 2D MoS2, the active regions are primarily concentrated at the edges, as the flat surface of MoS2 is not conducive for catalytic reactions. Additionally, MoS2 hinders the movement of electric charges. Integrating QDs with photocatalysts can assist in regulating the flow of charge carriers produced from light exposure [36].
In their study, Meng and his team successfully created heterostructures by combining MoS2 QDs with bismuth tungstate (Bi2WO6) structures using a straightforward sonication technique. These novel heterostructures demonstrated significantly improved efficacy in wastewater treatment and toxin removal compared to using MoS2 and Bi2WO6 materials independently [37-39].
The plate check method was used to test the germ-killing properties of MoS2-Bi2WO6 p-n heterojunction. The study found that the heterostructure had a lower amount of E. coli compared to pure Bi2WO6 and MoS2. The creation of this heterojunction enhanced the efficiency of capturing electrical carriers, resulting in a decrease in E. coli survival under visible light [40]. MoS2 QDs were found to effectively produce Reactive Oxygen Species, aiding in killing bacteria. Additionally, tests on living organisms showed its effectiveness in healing wounds [41].
Novel nanosystems containing MoS2 for wound healing applications
The most recent nano systems containing MoS2 nanoparticles are compiled in Table 1.
Table 1: Novel nanosystems incorporating MoS2 for wound healing
|
Sample |
Year |
Important Conclusion |
References |
|
MoS2-NS/Sericin |
2024 |
MoS2/Sericin shows superior wound healing properties under 808 nm irradiation |
[25] |
|
In situ 3D-bioprinting MoS2 |
2023 |
Accelerated wound healing, closure, reduced oxidative stress, and bacterial elimination are valuable for managing chronic wounds in diabetic patients |
[26] |
|
LAMC-MoS2@PDA |
2024 |
Hydrogels exhibit antibacterial effects, promote wound healing, and have clinical potential |
[20] |
|
Ag/MoS2 Nanozyme Hydrogel |
2023 |
An adhesion-enhanced self-healing nanozyme-modified hydrogel dressing provides promising biocompatible, antimicrobial surfaces |
[28] |
|
Biogenic capping agent l-cysteine (MoS2-cys NFs). |
2024 |
Modifying the surface of the flower-shaped MoS2 improves its ability to eliminate bacteria. This enhanced feature can be utilized for applications such as antibacterial coatings, water purification, and aiding in the healing of wounds |
[29] |
It is essential to include MoS2 nanoparticles in all wound dressings for wound healing. By 2024, the use of nanoparticles in wound dressings could help heal infected wounds by applying heat and radiation, as well as speeding up the healing process. Chronic injuries can be painful and burden healthcare systems worldwide [42-45]. A hydrogel made of MoS2 can remove harmful reactive oxygen species and promote quicker wound healing. It can also aid in the growth of blood vessels, showing promise for medical purposes [46-48].
CONCLUSION
The increasing prevalence of bacterial infections poses a significant threat to human health, despite advancements in modern medicine. Recently, there has been interest in utilizing MoS2-based structures to eliminate bacteria. By altering the shape of the band gap in MoS2 nanomaterials from circular to straight, they have been found to be more effective in sunlight and better at eradicating bacteria. The mechanisms by which MoS2 structures can eliminate bacteria include factors such as stretching, oxygen, and heat from sunlight. While research on MoS2 nanosheets and modified shapes has shown promising outcomes, exploration into MoS2 nanoflowers and quantum dots is still in its early stages. Furthermore, the combination of MoS2 with other two-dimensional materials has not been extensively studied. Understanding the potential harmful effects of MoS2-based materials, despite limited research in this area, is crucial. Ultimately, MoS2 has the potential to enhance the properties of biodegradable materials and develop antibacterial composites for medical applications. However, further research is necessary to fully comprehend the health and environmental risks associated with MoS2 nanomaterials.
REFERENCES
- Tan LF, Wang SP, Xu K, Liu TL, Liang P, Niu M, et al. (2016). Layered MoS2 hollow spheres for highly-efficient photothermal therapy of rabbit liver orthotopic transplantation tumors. Small. 12:2046–2055.
- Yuan Z, Tao BL, He Y, Liu J, Lin CC, Shen XK, et al. (2019). Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials. 217:119290.
- Wu HH, Yang R, Song BM, Han QS, Li JY, Zhang Y, et al. (2011). Biocompatible inorganic fullerene-like molybdenum disulfide nanoparticles produced by pulsed laser ablation in water. ACS Nano. 5:1276–1281.
- Karami MH, Abdouss M, Rahdar A, Pandey S, (2024). Graphene quantum dots: background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: an updated review. Inorg Chem Commun. 161:112032.
- Li J, Li M, Tang J, Li X, Zhang H, Zhang Y. (2008). Resonance light-scattering spectrometric study of interaction between enzyme and MPA-modified CdTe nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 70(3):514- 518.
- Hami Z. (2021). A Brief Review on Advantages of Nano-based Drug Delivery Systems. Ann Mil Health Sci Res. 19(1):e112274.
- Gelperina S, Kisich K, Iseman MD, Heifets L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 172(12):1487-1490.
- Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, et al. (2014). Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 8(10):9874-9883.
- Karami MH, Abdouss M. (2024). Cutting-edge tumor nanotherapy: Advancements in 5-fluorouracil Drug-loaded chitosan nanoparticles. Inorg Chem Communicat. 164:112430.
- Khan S, Rizvi SMD, Avaish M, Arshad M, Bagga P, Khan MS. (2015). A novel process for size controlled biosynthesis of gold nanoparticles using bromelain. Mater Lett. 159:373- 376.
- de Sousa IP, Cattoz B, Wilcox MD, Griffiths PC, Dalgliesh R, Rogers S, et al. (2015). Nanoparticles decorated with proteolytic enzymes, a promising strategy to overcome the mucus barrier. Eur J Pharm Biopharm. 97:257-264.
- Roy S, Haloi P, Choudhary R, Chawla S, Kumari M, Konkimalla VB, et al. (2023). Quaternary pullulan-functionalized 2D MoS2 glycosheets: A potent bactericidal nanoplatform for efficient wound disinfection and healing. ACS Appl Mater Interfaces. 15:24209–24227.
- Ataide JA, Gérios EF, Cefali LC, Fernandes AR, Teixeira MdC, Ferreira NR, et al. (2019). Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain–Chitosan Nanoparticles. Polymers. 11(10):1681.
- Kammona O, Kiparissides C. (2012). Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Control Release. 161(3):781-794.
- Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, et al. (2009). Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 9(4):325-341.
- Nagpal K, Singh SK, Mishra DN. (2010). Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull. 58(11):1423-1430.
- Karami MH, Pourmadadi M. (2023). Abdouss M, Kalaee MR, Moradi O, et al. Novel chitosan/γ-alumina/ carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int J Biol Macromol. 251:126280.
- Karami M H, Abdouss M. (2024). Assessing Particle Size and Surface Charge in Drug Carrier Nanoparticles for Enhanced Cancer Treatment: A Comprehensive Review Utilizing DLS and Zeta Potential Characterization. PSPRJ. 5(3): 000615.
- Brito AM, Oliveira V, Icimoto MY, Nantes-Cardoso IL. (2021). Collagenase activity of bromelain immobilized at gold nanoparticle interfaces for therapeutic applications. Pharmaceutics. 13(8):1143.
- Wang Y, Liu K, Huang K, Wei W, Huang Y, Dai H. (2024). Photothermal antibacterial MoS2 composited chitosan hydrogel for infectious wound healing. Biomater Adv. 156:213701.
- Karami MH, Abdouss M. (2024). Recent advances of carbon quantum dots in tumor imaging. Nanomed J. 11(1):13-35.
- Karami MH, Abdouss M, Maleki B. (2024). The State of the Art Metal Nanoparticles in Drug Delivery Systems: A Comprehensive Review Nanomed J.
- Kaushik R, Nandi S, Mandal M, Nath Gupta A. (2024). Biocompatible l-Cysteine-Capped MoS2 Nanoflowers for Antibacterial Applications: Mechanistic Insights. ACS Applied Nano Materials. 7(7):7753-7765.
- Hirche C, Almeland SK, Dheansa B, Fuchs P, Governa M, Hoeksema H, et al. (2020). Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns. 46(4):782-796.
- Qiu L, Duan L, Lin H, Wang M, Liang H, Peng G, et al. (2024). Multifunctional and Sprayable 2D MoS2/Silk Sericin Bio-Nanocomposite Dressings with Enhanced Photothermal Effect for Infected Wound Healing. Adv Fiber Mater.
- Ding X, Yu Y, Li W, Zhao Y. (2023). In situ 3D-bioprinting MoS2 accelerated gelling hydrogel scaffold for promoting chronic diabetic wound healing. Matter. 6(3):1000-1014.
- Karami MH, Abdouss M, Karami M. (2023). Evaluation of in vitro and ex vivo models for studying the effectiveness of vaginal drug systems in controlling microbe infections: A systematic review. Clin J Obst Gynecol. 6:201-215.
- Miranda ÍKSPB, Santana FR, Camilloto GP, Detoni CB, Souza FVD, de Magalhães Cabral-Albuquerque EC, et al. (2021). Development of membranes based on carboxymethyl cellulose/acetylated arrowroot starch containing bromelain extract carried on nanoparticles and liposomes. J Pharm Sci. 110(6):2372-2378.
- Bayat S, Zabihi AR, Farzad SA, Movaffagh J, Hashemi E, Arabzadeh S, et al. (2021). Evaluation of debridement effects of bromelain-loaded sodium alginate nanoparticles incorporated into chitosan hydrogel in animal models. Iran J Basic Med Sci. 24(10):1404.
- Karami M, Abdouss M, Kalaee M, Moradi O. (2023). Investigating the Antibacterial Properties of Chitosan Nanocomposites Containing Metal Nanoparticles For Using in Wound Healings: A Review Study. Basparesh, 2023.
- Karami MH, Abdouss M, Kalaee M, Moradi O. (2023). The application of chitosan-based nanocarriers in improving the release of the anticancer drug quercetin: a review study. Nano World. 19(70):21-11.
- Dabiri G, Damstetter E, Phillips T. (2016). Choosing a Wound Dressing Based on Common Wound Characteristics. Adv Wound Care. 5:32-41.
- Du X, Zhou J, Shi J, Xu B. (2015). Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem Rev. 115:13165-13307.
- Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA, et al. (2020). Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers. 12:2702.
- Rebers L, Reichsöllner R, Regett S, Tovar G, Borchers K, Baudis S, et al. (2021). Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci Rep. 11:3256.
- Morello G, Polini A, Scalera F, Rizzo R, Gigli G, Gervaso F. (2021). Preparation and Characterization of Salt-Mediated Injectable Thermosensitive Chitosan/Pectin Hydrogels for Cell Embedding and Culturing. Polymers. 13:2674.
- O'Meara S, Martyn-St James M, Adderley UJ. (2015). Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev. 2015:CD010182.
- Chang NS, Lin R, Sze CI, Aqeilan RI. (2019). Editorial: WWDomain Proteins in Signaling, Cancer Growth, Neural Diseases, and Metabolic Disorders. Front Oncol. 9:719.
- Brown BN, Badylak SF. (2014). Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 163(4):268-285.
- Zhang X, Tan B, Wu Y, Zhang M, Liao J. (2021). A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers. 13(12):2100.
- Verhelst S, Bonger KM, Willems LI. (2020). Bioorthogonal Reactions in Activity-Based Protein Profiling. Molecules. 25(26):5994.
- Gupta A, Briffa SM, Swingler S, Gibson H, Kannappan V, Adamus G, et al. (2020). Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules. 21(5):1802-1811.
- Nešović K, Janković A, Kojić V, Vukašinović-Sekulić M, Perić-Grujić A, Rhee KY, et al. (2018). Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—Synthesis, biological and physicochemical properties and silver release kinetics. Compos Part B Eng. 154:175-185.
- Sun A, He X, Li L, Li T, Liu Q, Zhou X, et al. (2020). An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater. 12:25.
- Echalier C, Laurine V, Martinez J, Mehdi A, Gilles S. (2019). Chemical cross-linking methods for cell encapsulation in hydrogels. Materials Today Communications. 20:100536.
- Xin H, Biswas N, Li P, Zhong C, Chan TC, Nudleman E, et al. (2021). Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc Natl Acad Sci U S A. 118(5):e1921252118.
- Gelperina S, Kisich K, Iseman MD, Heifets L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 172(12):1487-1490.
- Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, et al. (2014). Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS nano. 8(10):9874-9883.
 Abstract
Abstract  PDF
PDF